
 中文核心期刊
中文核心期刊华东师范大学学报(自然科学版) ›› 2023, Vol. 2023 ›› Issue (1): 82-94.doi: 10.3969/j.issn.1000-5641.2022.00.009
收稿日期:2022-07-11
接受日期:2022-08-01
出版日期:2023-01-25
发布日期:2023-01-07
通讯作者:
吴鹏
E-mail:yma@chem.ecnu.edu.cn;pwu@chem.ecnu.edu.cn
作者简介:马 跃, 男, 博士后, 研究方向为沸石基催化材料. E-mail: 基金资助:
Yue MA( ), Hao XU, Yueming LIU, Kun ZHANG, Peng WU*(
), Hao XU, Yueming LIU, Kun ZHANG, Peng WU*( ), Mingyuan HE
), Mingyuan HE
Received:2022-07-11
Accepted:2022-08-01
Online:2023-01-25
Published:2023-01-07
Contact:
Peng WU
E-mail:yma@chem.ecnu.edu.cn;pwu@chem.ecnu.edu.cn
摘要:
面向C1分子及低碳烷烃活化脱氢制备C2和C3烯烃等重要化工过程, 设计高效、稳定的负载型金属催化剂, 防止金属物种在苛刻的制备和反应条件下烧结成大尺寸纳米颗粒是一个关键的挑战. 具有均匀亚纳米尺寸孔道和丰富三维晶体结构的多孔沸石分子筛, 已被证明是将金属团簇封装在其孔道内以制备高效稳定金属催化剂的理想载体. 更重要的是, 亚纳米金属团簇和沸石骨架原子之间的相互作用可以调节其几何和电子结构. 研发沸石孔道限域的亚纳米团簇型金属催化剂旨在利用这种联合的约束效应, 诱导金属物种与具有活性位点的沸石骨架间的协同作用, 进一步提升复合催化剂的催化性能, 应用于多种催化反应过程. 本文主要介绍了几种典型的方法制备沸石孔道限域的亚纳米团簇型金属催化剂, 以及在CO2和炔烃选择性加氢、甲酸分解产氢、氨硼烷水解产氢及丙烷脱氢制丙烯反应过程中的催化应用.
中图分类号:
马跃, 徐浩, 刘月明, 张坤, 吴鹏, 何鸣元. 沸石孔道限域亚纳米金属团簇催化剂研究进展[J]. 华东师范大学学报(自然科学版), 2023, 2023(1): 82-94.
Yue MA, Hao XU, Yueming LIU, Kun ZHANG, Peng WU, Mingyuan HE. Review of zeolite-confined subnanometric cluster catalysts[J]. Journal of East China Normal University(Natural Science), 2023, 2023(1): 82-94.
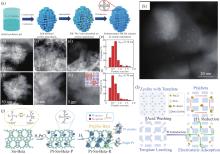
图1
浸渍、离子交换和模板剂引导法制备沸石孔道限域型金属催化剂 注: (a) 通过浸渍将亚纳米级Rh-Ru团簇封装在自柱撑MFI纳米片中的合成方法示意图[21]; (b)—(d) Rh0.8Ru0.2/SP-S-1和 (f)—(h) Rh0.8Ru0.2/SP-ZSM-5-100的球差校正扫描透射电子显微镜照片[21]; (e) Rh0.8Ru0.2/SP-S-1和 (i) Rh0.8Ru0.2/SP-ZSM-5-100催化剂中金属颗粒的尺寸分布[21]; (j) Sn-Beta封装单原子Pt及亚纳米团簇(Pt@Sn-Beta)的合成路线示意图[38]; (k) Pt/CHA-8_exc的STEM图像(0.31 wt% Pt)[53]; (l) 沸石孔道内封装金属团簇的模板剂引导方法路线图[55]."
图2
原位水热法制备沸石孔道限域型金属催化剂 注: (a)原位水热法合成Pd-M(OH)2@S-1示意图[20]; (b), (c) 0.8Pd0.2Ni(OH)2@S-1的球差校正的STEM图像[20]; (d) Rh@S-1-H催化剂制备过程示意图[31]; (e) Rh@S-1-H沿[011]方向的球差校正HAADF-STEM图像及同一投影方向上的模型示意图[31]; (f)通过两种不同的合成方法将Pt-Zn封装到S-1沸石上的过程示意图[32]; (g), (h) PtZn4@S-1-H的高分辨率球差校正HAADF-STEM图像[32]; (i)原位水热法合成Pt@MFI和K-Pt@MFI催化剂的流程示意图[42]; K-PtSn@MFI样品在(j) [010]方向和(k) 倾斜-[010]方向的球差校正HAADF-STEM图像[42]."
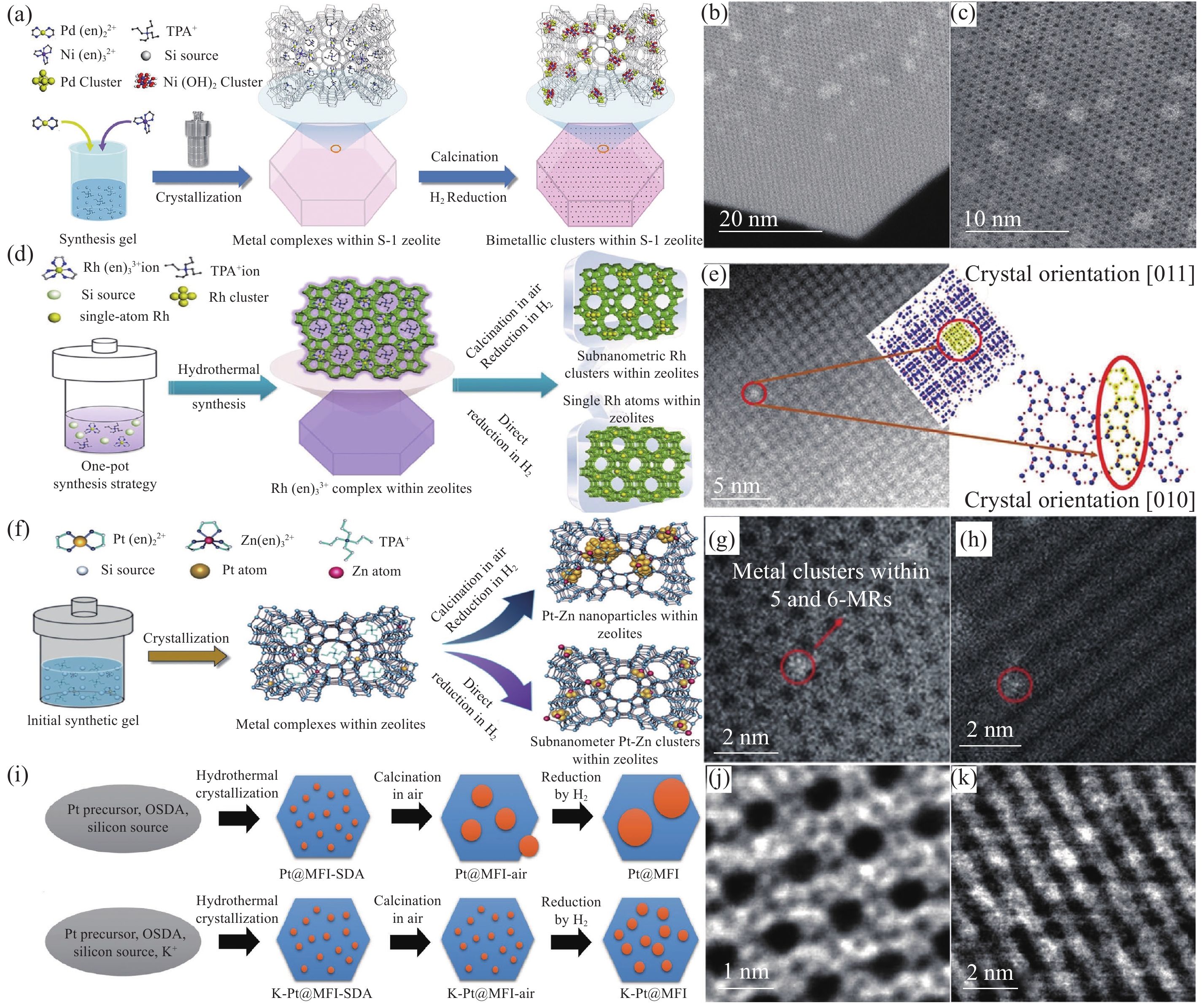

图4
不同分子筛限域金属催化剂的选择性加氢性能 注: (a) Rh@HZSM-5; (b) Rh@S-1; (c) Rh@S-1-OH及 (d) Rh@KZSM-5 材料的CO2加氢催化性能 (反应条件为催化剂0.5 g、1 MPa原料气压力、nCO2/nH2/nAr = 1/3/1、进料速度为30 mL·min–1[39]); (e) 各种催化剂上的CO选择性(Sel.)与CO2转化率(Conv.)的相关性[39]; (f) Rh@HZSM-5和Rh@S-1催化剂上CO和CH4生成速率与反应温度的相关性[39]; (g) 各种催化剂上CO2加氢生成甲酸酯的速率[29]; (h) PdMn0.6@S-1催化CO2加氢的循环稳定性试验[29](反应条件为催化剂5 mg、1.5 mol/L三乙胺溶液2 mL、PH2/PCO2 = 20 /20 (P为压力, bar)、25℃[29]); (i) Pd@SOD和Pd/SOD催化剂上乙炔转化率和乙烯、乙烷选择性 (反应条件为气体每小时空速(GHSV) = 30000 h–1、150℃、VH2/VC2H2 = 10/1[40](V为流速, mL·min–1, 下同)); (j) 通过DFT计算得到的乙炔在Pd@SOD (红色) 和Pd/SOD (黑色) 表面上加氢生成乙烷的势能图[40]."
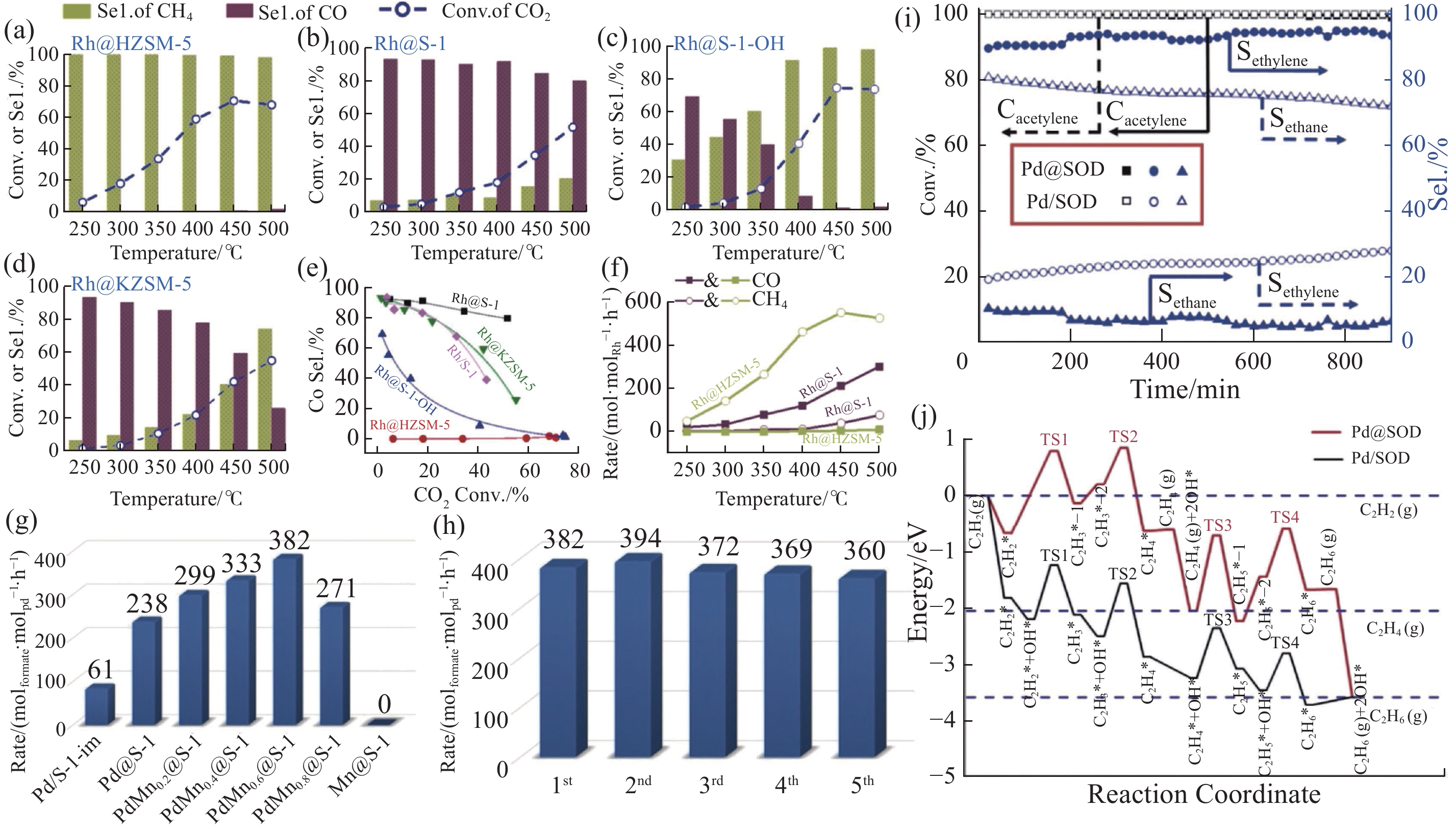

图5
不同分子筛限域金属催化剂的甲酸或氨硼烷产氢性能 注: (a) 60℃下, Pd/C、Pd@S-1、Ni(OH)2@S-1和Pd-Ni(OH)2@S-1催化剂在2 mol/L甲酸溶液中反应释放的气体(CO2 + H2)体积与脱氢时间的关系(nPd/nFA = 0.012)[20]; (b) 0.8Pd0.2Ni(OH)2@S-1催化剂在不同温度下分解2mol/L甲酸溶液生成H2的初始TOF值[20]; (c) 60℃ (nPd/nFA = 0.012) 反应条件下, 不同催化剂上甲酸分解(2.0 mol/L, 1.5 mL)产生的气体随时间的变化[29]; (d) 60℃反应条件下, 在PdMn0.6@S-1催化剂上进行甲酸分解(2.0 mol/L, 1.5 mL)的循环稳定性试验[29]; (e) Rh/SP-ZSM-5-100、Ru/SP-ZSM-5-100和不同的RhxRu1-x/SP-ZSM-5-100催化剂在25℃(nmetal/nAB = 0.001) 条件下催化甲酸(1 mol/L)水解产生的H2体积随时间的变化[21]; (f) 25℃下, 在Rh0.8Ru0.2/SP-ZSM-5-100催化剂上进行氨硼烷水解的循环试验[21]."
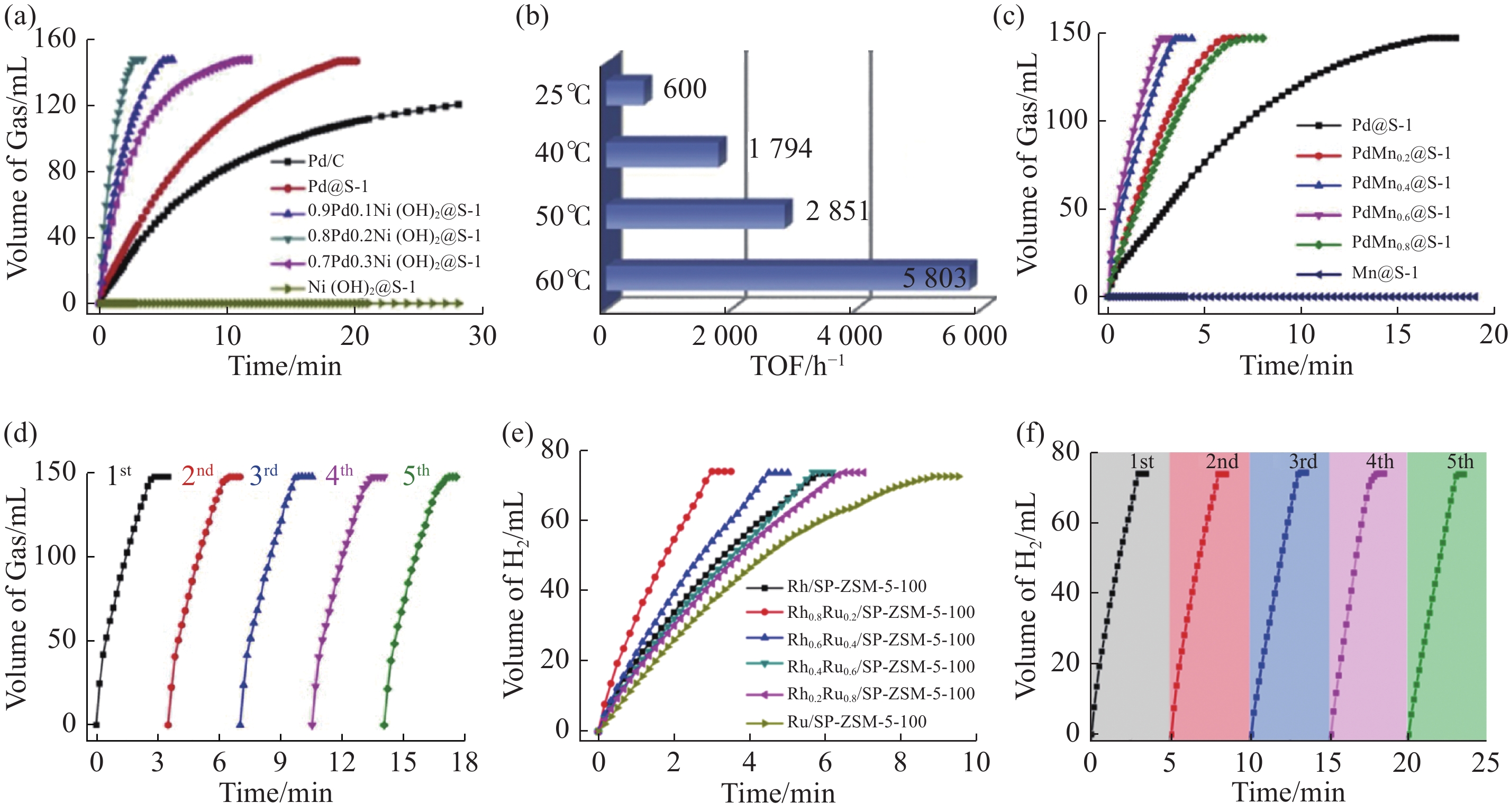

图6
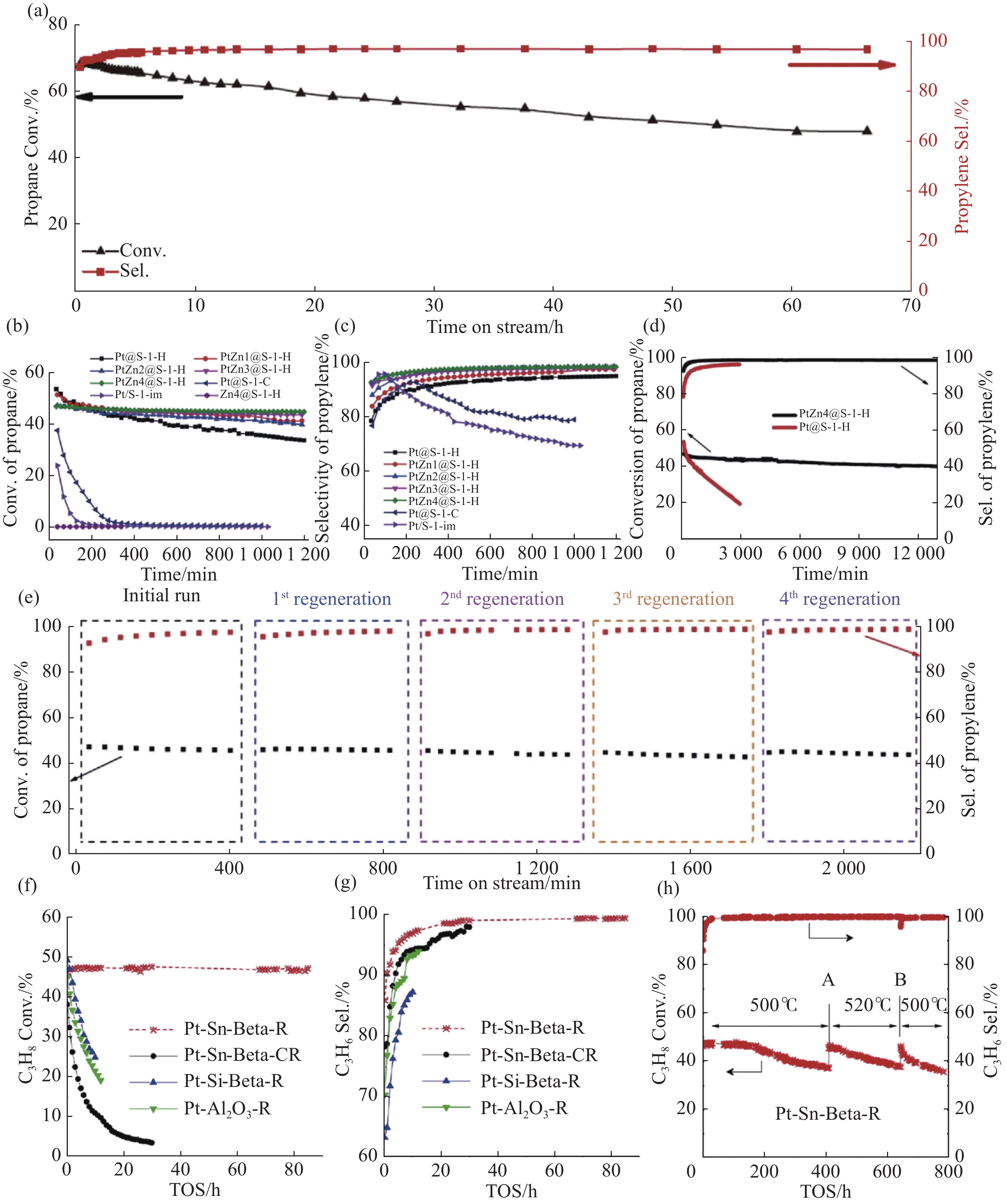
不同分子筛限域金属催化剂的丙烷脱氢性能 注: (a) 600℃下, K-PtSn@MFI催化丙烷在脱氢的第一个反应循环[42] (反应条件为320 mg催化剂, 原料为丙烷(5 mL·min–1)和N2 (16 mL·min–1)的混合气, 常压[21]); 不同催化剂上的 (b) 丙烷转化率和 (c) 丙烯选择性[32]; (d) PtZn4@S-1-H和Pt@S-1-H催化剂上PDH反应的长期稳定性试验[32]; (e) 多次再生循环后丙烷在PtZn4@S-1-Cs催化剂上的转化率和丙烯的选择性[32] (反应条件为0.3 g 催化剂与1 g石英砂混合, 常压, VC3H8/VN2 = 10/30[40], WHSV = 3.6 h–1, 550℃[32]); 500℃下, 不同催化剂催化丙烷脱氢反应的 (f) 丙烷转化率和 (g) 丙烯选择性随反应时间 (TOS) 变化的结果[38] (反应条件为500℃温度下, H2预还原2 h, 0.3 g 催化剂, W/F (催化剂重量W与进料气体流速F的比值) = 1.8 g·s·cm–3, nN2/nC3H8 = 19, 常压[38]); (h) 不同处理条件下Pt-Sn-Beta-R催化剂的丙烷脱氢反应结果[38] (反应条件为500℃的温度下, H2预还原2 h, 0.3 g催化剂, W/F = 1.8 g·s·cm–3, nN2/nC3H8 = 19, 常压, 不同处理方法为(A) 将脱氢反应温度从500℃提高到520℃, (B) 500℃的空气中烧积碳处理2 h[38])."
| 1 | ZHANG Q, YU J, CORMA A. Applications of zeolites to C1 chemistry: Recent advances, challenges, and opportunities. Advanced Materials, 2020, 32 (44): 2002927. |
| 2 | LIU L, CORMA A. Metal catalysts for heterogeneous catalysis: From single atoms to nanoclusters and nanoparticles. Chemical Reviews, 2018, 118 (10): 4981- 5079. |
| 3 | DENG J, DENG D, BAO X. Robust catalysis on 2D materials encapsulating metals: Concept, application, and perspective. Advanced Materials, 2017, 29 (43): 1606967. |
| 4 | WANG A, LI J, ZHANG T. Heterogeneous single-atom catalysis. Nature Reviews Chemistry, 2018, 2 (6): 65- 81. |
| 5 | GATES B. Atomically dispersed supported metal catalysts: Seeing is believing. Trends in Chemistry, 2019, 1 (1): 99- 110. |
| 6 | GATES B, FLYTZANI-STEPHANOPOULOS M, DIXON D, et al. Atomically dispersed supported metal catalysts: Perspectives and suggestions for future research. Catalysis Science & Technology, 2017, 7 (19): 4259- 4275. |
| 7 | LI Z J, WANG D , WU Y E, et al. Recent advances in the precise control of isolated single-site catalysts by chemical methods. National Science Review, 2018, 5 (5): 673- 689. |
| 8 | QIN R X, LIU P X, FU G, et al. Strategies for stabilizing atomically dispersed metal catalysts. Small Methods, 2018, 2 (1): 1700286. |
| 9 | LI J, ZHANG K, WANG N, et al. Research progress of zeolite-encaged single-atom metal catalysts. Chemical Journal of Chinese Universities, 2022, 43 (5): 20220032. |
| 10 | LIU L, MEIRA D, ARENAL R, et al. Determination of the evolution of heterogeneous single metal atoms and nanoclusters under reaction conditions: Which are the working catalytic sites?. ACS Catalysis, 2019, 9 (12): 10626- 10639. |
| 11 | TYO E, VAJDA S. Catalysis by clusters with precise numbers of atoms. Nature Nanotechnology, 2015, 10 (7): 577- 588. |
| 12 | WAN X, WU H, GUAN B, et al. Confining sub-nanometer Pt clusters in hollow mesoporous carbon spheres for boosting hydrogen evolution activity. Advanced Materials, 2020, 32 (7): 1901349. |
| 13 | MON M, RIVERO-CRESPO M, FERRANDO-SORIA J, et al. Synthesis of densely packaged, ultrasmall PtO2 clusters within a thioether-functionalized MOF: Catalytic activity in industrial reactions at low temperature . Angewandte Chemie-International Edition, 2018, 57 (21): 6186- 6191. |
| 14 | ZHANG J, YE J, HUANG T, et al. Conversion of triphenylphosphine oxide to organophosphorus via selective cleavage of C-P, O-P, and C-H bonds with sodium. Communications Chemistry, 2020, (3): 1- 9. |
| 15 | SUN Q, WANG N, YU J. Advances in catalytic applications of zeolite-supported metal catalysts. Advanced Materials, 2021, 33 (51): 2104442. |
| 16 | GOEL S, WU Z, ZONES S, et al. Synthesis and catalytic properties of metal clusters encapsulated within small-pore (SOD, GIS, ANA) zeolites. Journal of the American Chemical Society, 2012, 134 (42): 17688- 17695. |
| 17 | WANG C, WANG L, ZHANG J, et al. Product selectivity controlled by zeolite crystals in biomass hydrogenation over a palladium catalyst. Journal of the American Chemical Society, 2016, 138 (25): 7880- 7883. |
| 18 | SUN Q, WANG N, BAI R, et al. Synergetic effect of ultrasmall metal clusters and zeolites promoting hydrogen generation. Advanced Science, 2019, 6 (10): 1802350. |
| 19 | LIU L, DÍAZ U, ARENAL R, et al. Generation of subnanometric platinum with high stability during transformation of a 2D zeolite into 3D. Nature Materials, 2017, 16 (1): 132- 138. |
| 20 | SUN Q, WANG N, BING Q, et al. Subnanometric hybrid Pd-M(OH)2, M = Ni, Co, clusters in zeolites as highly efficient nanocatalysts for hydrogen generation . Chem, 2017, 3 (3): 477- 493. |
| 21 | WANG N, SUN Q, ZHANG T, et al. Impregnating subnanometer metallic nanocatalysts into self-pillared zeolite nanosheets. Journal of the American Chemical Society, 2021, 143 (18): 6905- 6914. |
| 22 | WANG N, SUN Q, BAI R, et al. In situ confinement of ultrasmall Pd clusters within nanosized silicalite-1 zeolite for highly efficient catalysis of hydrogen generation. Journal of the American Chemical Society, 2016, 138 (24): 7484- 7487. |
| 23 | LIU L, WANG N, ZHU C, et al. Direct imaging of atomically dispersed molybdenum that enables location of aluminum in the framework of zeolite ZSM-5. Angewandte Chemie-International Edition, 2020, 59 (2): 819- 825. |
| 24 | WANG C, LIU Z, WANG L, et al. Importance of zeolite wettability for selective hydrogenation of furfural over Pd@zeolite catalysts. ACS Catalysis, 2018, 8 (1): 474- 481. |
| 25 | QU Z, SUN Q. Advances in zeolite-supported metal catalysts for propane dehydrogenation. Inorganic Chemistry Frontiers, 2022, 9 (13): 3095- 3115. |
| 26 | ZHAO C, LERCHER J. Upgrading pyrolysis oil over Ni/HZSM-5 by cascade reactions. Angewandte Chemie-International Edition, 2012, 51 (24): 5935- 5940. |
| 27 | GATES B. Supported metal clusters: Synthesis, structure, and catalysis. Chemical Reviews, 1995, 95 (3): 511- 522. |
| 28 | WHITE R, LUQUE R, BUDARIN V, et al. Supported metal nanoparticles on porous materials. Methods and applications. Chemical Society Reviews, 2009, 38 (2): 481- 494. |
| 29 | SUN Q, CHEN B, WANG N, et al. Zeolite-encaged Pd-Mn nanocatalysts for CO2 hydrogenation and formic acid dehydrogenation . Angewandte Chemie-International Edition, 2020, 59 (45): 20183- 20191. |
| 30 | SUN Q, WANG N, XU Q, et al. Nanopore-supported metal nanocatalysts for efficient hydrogen generation from liquid-phase chemical hydrogen storage materials. Advanced Materials, 2020, 32 (44): 2001818. |
| 31 | SUN Q, WANG N, ZHANG T, et al. Zeolite-encaged single-atom rhodium catalysts: Highly-efficient hydrogen generation and shape-selective tandem hydrogenation of nitroarenes. Angewandte Chemie-International Edition, 2019, 58 (51): 18570- 18576. |
| 32 | SUN Q, WANG N, FAN Q, et al. Subnanometer bimetallic platinum-zinc clusters in zeolites for propane dehydrogenation. Angewandte Chemie-International Edition, 2020, 59 (44): 19450- 19459. |
| 33 | CUI T, KE W, ZHANG W, et al. Encapsulating palladium nanoparticles inside mesoporous MFI zeolite nanocrystals for shape-selective catalysis. Angewandte Chemie-International Edition, 2016, 55 (32): 9178- 9182. |
| 34 | ZHANG J, WANG L, ZHANG B, et al. Sinter-resistant metal nanoparticle catalysts achieved by immobilization within zeolite crystals via seed-directed growth. Nature Catalysis, 2018, 1 (7): 540- 546. |
| 35 | LI S, TUEL A, LAPRUNE D, et al. Transition-metal nanoparticles in hollow zeolite single crystals as bifunctional and size-selective hydrogenation catalysts. Chemistry of Materials, 2015, 27 (1): 276- 282. |
| 36 | GOEL S, ZONES S, IGLESIA E. Encapsulation of metal clusters within MFI via interzeolite transformations and direct hydrothermal syntheses and catalytic consequences of their confinement. Journal of the American Chemical Society, 2014, 136 (43): 15280- 15290. |
| 37 | WANG Y, HU Z, TIAN W, et al. Framework-confined Sn in Si-beta stabilizing ultra-small Pt nanoclusters as direct propane dehydrogenation catalysts with high selectivity and stability. Catalysis Science & Technology, 2019, 9 (24): 6993- 7002. |
| 38 | MA Y, CHEN X, GUAN Y, et al. Skeleton-Sn anchoring isolated Pt site to confine subnanometric clusters within *BEA topology. Journal of Catalysis, 2021, 397, 44- 57. |
| 39 | WANG C, GUAN E, WANG L, et al. Product selectivity controlled by nanoporous environments in zeolite crystals enveloping rhodium nanoparticle catalysts for CO2 hydrogenation . Journal of the American Chemical Society, 2019, 141 (21): 8482- 8488. |
| 40 | WANG S, ZHAO Z, CHANG X, et al. Activation and spillover of hydrogen on sub-1 nm palladium nanoclusters confined within sodalite zeolite for the semi-hydrogenation of alkynes. Angewandte Chemie-International Edition, 2019, 58 (23): 7497- 7880. |
| 41 | ZHU J, OSUGA R, ISHIKAWA R, et al. Ultrafast encapsulation of metal nanoclusters into MFI zeolite in the course of its crystallization: Catalytic application for propane dehydrogenation. Angewandte Chemie-International Edition, 2020, 59 (44): 19669- 19674. |
| 42 | LIU L, LOPEZ-HARO M, LOPES C, et al. Regioselective generation and reactivity control of subnanometric platinum clusters in zeolites for high-temperature catalysis. Nature Materials, 2019, 18 (8): 866- 873. |
| 43 | WANG Y, HU Z, LV X, et al. Ultrasmall PtZn bimetallic nanoclusters encapsulated in silicalite-1 zeolite with superior performance for propane dehydrogenation. Journal of Catalysis, 2020, 385, 61- 69. |
| 44 | LIU L, LOPEZ-HARO M, LOPES C, et al. Structural modulation and direct measurement of subnanometric bimetallic PtSn clusters confined in zeolites. Nature Catalysis, 2020, 3 (8): 628- 638. |
| 45 | CUI W, HU T. Incorporation of active metal species in crystalline porous materials for highly efficient synergetic catalysis. Small, 2021, 17 (22): 2003971. |
| 46 | SERNA P, GATES B. Molecular metal catalysts on supports: Organometallic chemistry meets surface science. Accounts of Chemical Research, 2014, 47 (8): 2612- 2620. |
| 47 | ZEČEVIĆ J, EERDEN A, FRIEDRICH H, et al. Heterogeneities of the nanostructure of platinum/zeolite Y catalysts revealed by electron tomography. ACS Nano, 2013, 7 (4): 3698- 3705. |
| 48 | MUNNIK P, JONGH P, JONG K. Recent developments in the synthesis of supported catalysts. Chemical Reviews, 2015, 115 (14): 6687- 6718. |
| 49 | WANG N, SUN Q, YU J. Ultrasmall metal nanoparticles confined within crystalline nanoporous materials: A fascinating class of nanocatalysts. Advanced Materials, 2019, 31 (1): 1803966. |
| 50 | HUANG W, ZHANG S, TANG Y, et al. Low-temperature transformation of methane to methanol on Pd1O4 single sites anchored on the internal surface of microporous silicate . Angewandte Chemie-International Edition, 2016, 55 (43): 13441- 13445. |
| 51 | WEI L, GRÉNMAN H, HAIJE W, et al. Sub-nanometer ceria-promoted Ni 13X zeolite catalyst for CO2 methanation . Applied Catalysis A: General, 2021, 612, 118012. |
| 52 | GRAAF J, DILLEN A, JONG K, et al. Preparation of highly dispersed Pt particles in zeolite Y with a narrow particle size distribution: Characterization by hydrogen chemisorption, TEM, EXAFS spectroscopy, and particle modeling. Journal of Catalysis, 2001, 203 (2): 307- 321. |
| 53 | MOLINER M, GABAY J, KLIEWER C, et al. Trapping of metal atoms and metal clusters by chabazite under severe redox stress. ACS Catalysis, 2018, 8 (10): 9520- 9528. |
| 54 | MOLINER M, GABAY J, KLIEWER C, et al. Reversible transformation of Pt nanoparticles into single atoms inside high-silica chabazite zeolite. Journal of the American Chemical Society, 2016, 138 (48): 15743- 15750. |
| 55 | TIAN Y, DUAN H, ZHANG B, et al. Template guiding for the encapsulation of uniformly subnanometric platinum clusters in Beta-zeolites enabling high catalytic activity and stability. Angewandte Chemie-International Edition, 2021, 60 (40): 21713- 21717. |
| 56 | CUNDY C, COX P. The hydrothermal synthesis of zeolites: Precursors, intermediates and reaction mechanism. Microporous and Mesoporous Materials, 2005, 82 (1/2): 1- 78. |
| 57 | CHOI M, WU Z, IGLESIA E. Mercaptosilane-assisted synthesis of metal clusters within zeolites and catalytic consequences of encapsulation. Journal of the American Chemical Society, 2010, 132 (26): 9129- 9137. |
| 58 | LIU L, ARENAL R, MEIRA D, et al. Generation of gold nanoclusters encapsulated in an MCM-22 zeolite for the aerobic oxidation of cyclohexane. Chemical Communications, 2019, 55 (11): 1607- 1610. |
| 59 | TEDSREE K, LI T, JONES S, et al. Hydrogen production from formic acid decomposition at room temperature using a Ag-Pd core-shell nanocatalyst. Nature Nanotechnology, 2011, 6 (5): 302- 307. |
| 60 | GRASEMANN M, LAURENCZY G. Formic acid as a hydrogen source-recent developments and future trends. Energy & Environmental Science, 2012, 5 (8): 8171- 8181. |
| 61 | GRAETZ J. New approaches to hydrogen storage. Chemical Society Reviews, 2009, 38 (1): 73- 82. |
| 62 | SONG F, ZHU Q, TSUMORI N, et al. Diamine-alkalized reduced graphene oxide: Immobilization of sub-2 nm palladium nanoparticles and optimization of catalytic activity for dehydrogenation of formic acid. ACS Catalysis, 2015, 5 (9): 5141- 5144. |
| 63 | JIANG K, XU K, ZOU S, et al. B-doped Pd catalyst: Boosting room-temperature hydrogen production from formic acid-formate solutions. Journal of the American Chemical Society, 2014, 136 (13): 4861- 4864. |
| 64 | SATTLER J, RUIZ-MARTINEZ J, SANTILLAN-JIMENEZ E, et al. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chemical Reviews, 2014, 114 (20): 10613- 10653. |
| [1] | 黄民生, 杨银川, 崔贺, 杨乐, 何岩, 曹承进. 新型悬浮填料强化硝化作用试验研究[J]. 华东师范大学学报(自然科学版), 2019, 2019(6): 115-122. |
| [2] | 周 栋;陈振楼;毕春娟;王 骏;林守民;祁莹莹. 沸石和麦饭石组合滤料对城市降雨径流氮磷去除效率的研究[J]. 华东师范大学学报(自然科学版), 2011, 2011(1): 185-193. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||