环境污染是当今社会面临的重大问题之一, 半导体光催化剂作为一种新型的绿色科技物质, 能够降解有机污染物(如甲基蓝、罗丹明B、苯酚等), 一直是人们关注和研究的重要课题之一.以锐钛矿型TiO
MOFs是一种通过多齿状有机基团连接金属离子或基团形成的一维、二维或三维的空间网格结构的金属有机复合物[3-4], 因其具有大的孔隙率、大比表面积、可变孔径和可变的功能基团而在气体存储、催化领域、光电领域等方面受到广泛关注.在MOFs材料中MIL-125(Ti)是一个典型代表, 它是以循环八聚物TiO
因此, 可以将MIL-125(Ti)与BiOI进行复合, 形成一种能带相匹配的具有异质结结构的复合物.这样不仅能保留MIL-125(Ti)比表面积大的优点, 还能够有效吸收可见光, 从而在可见光的照射下有效地降解有机污染物[5-7].本文通过一步共沉淀法制备异质结结构MIL-125(Ti)/BiOI, 探究该催化剂在可见光辐射下对罗丹明B的光催化降解效果, 并结合其表征, 从机理上进行分析.
1 实验部分 1.1 实验试剂与仪器试剂:五水硝酸铋、碘化钾、乙二醇、柠檬酸、对苯二甲酸(H
仪器: BL-GHX-V型光化学反应仪(Photochemical Reactions Instrument); U-3900型紫外可见分光光度计(UV-Vis); D/MAX2500PC型X射线衍射仪(XRD); Hitachi S4480型扫描电子显微镜(SEM); Fluoromax-4型荧光分光光度计(PL); N
量取216mL DMF, 甲醇24mL, 倒入500mL的烧杯中, 磁力搅拌30min; 称取12.0g(0.0722mol)H
称取0.3520g(0.7256mmol)Bi(NO
称取80mg MIL-125(Ti)/BiOI样品于石英管中, 加入转子, 称取80mg/L的RhB(罗丹明B)溶液10g, 加去离子水稀释到10mg/L.暗反应30min, 使其达到吸附-脱附平衡, 再将其超声15min, 防止因颗粒团聚对实验结果产生影响[9-10].在光化学反应仪中, 500W氙灯辐射下, 光催化反应90min(前50min, 每10min取1个样, 后面每20min取1个样, 共8个样).离心分离(8000r/min)5min, 取上清液, 用紫外可见分光光度计测试罗丹明B的吸收强度, 在最强吸收峰处计算罗丹明B的归一化浓度.比较不同掺杂比例(Ti:Bi)的降解效果.归一化浓度的计算公式为
| $ \begin{align*} Y=\frac{C}{C_{0}}\times/100\%, \end{align*} $ |
其中,
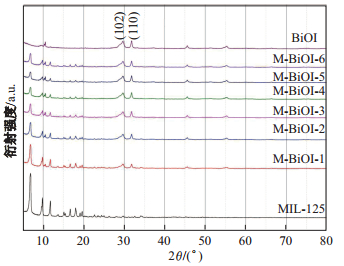
由图 1可知, M-BiOI-
|
图 1 MIL-125(Ti)、BiOI和M-BiOI- |
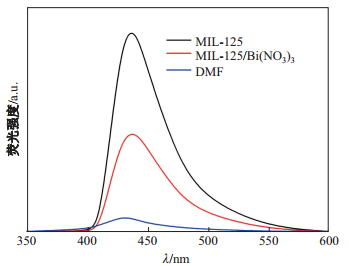
用260nm紫外光做激发光源, 分别测试DMF(空白), 5mmol/L MIL-125(Ti)的DMF溶液, 5mmol/L MIL-125(Ti)/Bi(NO
|
图 2 DMF、MIL-125(Ti)的DMF溶液和MIL-125(Ti)/Bi(NO |
一般而言, 由于半导体材料对能量高于其吸收限的光子有很强的吸收, 能产生额外的光生电子-空穴对, 这些载流子一边向材料表面扩散, 一边通过各种复合机制复合, 而PL的强度与复合的概率成正比, PL强度越低, 光生电子-空穴复合概率越低[13].如图 2所示, 5mmol MIL-125(Ti)/Bi(NO
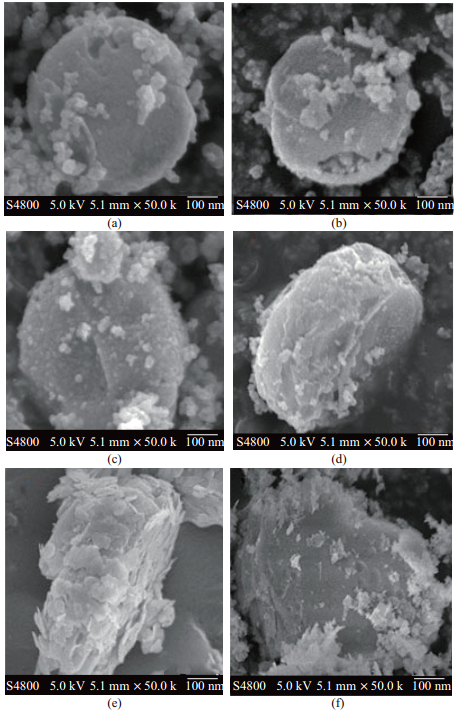
制备的M-BiOI-
|
图 3 M-BiOI-1 (a)、M-BiOI-2 (b)、M-BiOI-3 (c)、M-BiOI-4 (d)、M-BiOI-5 (e)、M-BiOI-6 (f)的SEM照片 Fig.3 SEM images of M-BiOI-1 (a), M-BiOI-2 (b), M-BiOI-3 (c), M-BiOI-4 (d), M-BiOI-5 (e), M-BiOI-6 (f) |
|
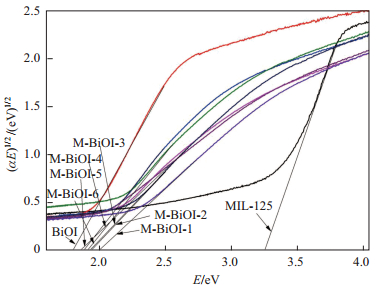
图 7 MIL-125(Ti)、BiOI和M-BiOI- |
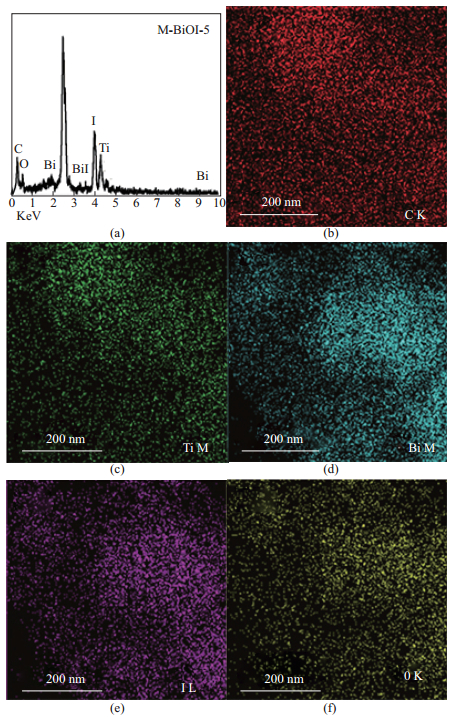
图 4为M-BiOI-5(根据图 3的SEM照片, 可知M-BiOI-5中BiOI在MIL-125(Ti)表面分布最均匀, 所以这里选择M-BiOI-5做EDX图谱分析)的EDX图谱及各元素映射图像.
|
图 4 4 M-BiOI-5的EDX(a)以及各元素的元素映射图像(b)-(f) Fig.4 (a) EDX spectrum, (b)-(f) corresponding elemental mapping images of M-BiOI-5 |
由图 4(a)可以很直观地看出M-BiOI-5中C、Ti、Bi、I、O等元素含量的对比情况, 同时可以看出没有其他的元素, 说明制备的样品纯度很高. 图 4(b)至图 4(f)分别为C、Ti、Bi、I、O的元素映射图像, 可以看出在M-BiOI-5中, BiOI均匀地分布在MIL-125(Ti)的表面, 这进一步印证了BiOI和MIL-125(Ti)形成了比较好的微观结构.
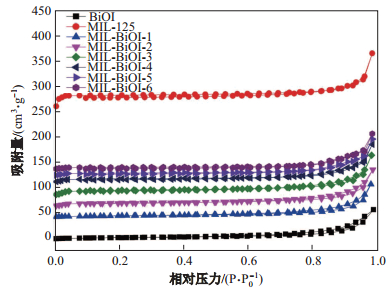
2.1.5 BET测试图 5为BiOI、MIL-125(Ti)、M-BiOI-
|
图 5 BiOI、MIL-125 (Ti)和M-BiOI- |
表 1所示为BiOI、MIL-125(Ti)、M-BiOI-
|
表 1 BiOI、MIL-125 (Ti)和M-BiOI- |
当掺杂BiOI后, M-BiOI-
|
图 8 MIL-125(Ti)、BiOI和M-BiOI- |
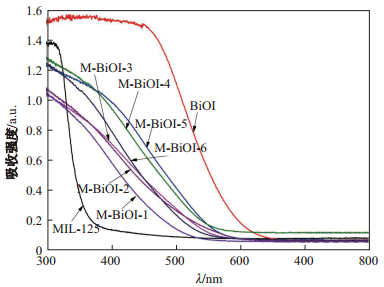
图 6为MIL-125(Ti)、BiOI和M-BiOI-
|
图 6 MIL-125(Ti)、BiOI和M-BiOI- |
对于晶体半导体, 光吸收和能带遵循光电效应, 公式[18]为
| $ \begin{align*} \alpha hv =A(hv - E_{\rm g} )^{n/2}, \end{align*} $ |
其中,
由图 7可以看出, BiOI的能带大概为1.8eV, 与文献[21]中1.72eV基本相符; M-BiOI-
图 8为MIL-125(Ti)、BiOI和M-BiOI-
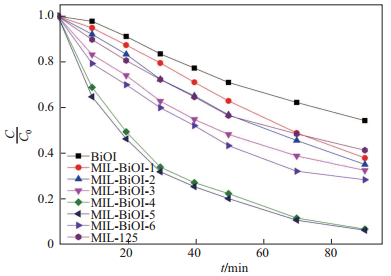
为更好地理解M-BiOI-
| $ \begin{align*} {\rm ln}\frac{C_{0}}{C}=k_{\rm app}t, \end{align*} $ |
其中,
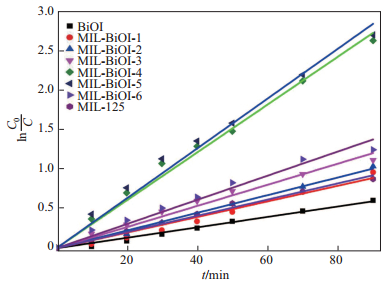
|
图 9 MIL-125(Ti)、BiOI和M-BiOI- |
|
表 2 MIL-125(Ti)、BiOI和M-BiOI- |
由表 2可知, 对应样品的动力学反应速率常数随着BiOI的掺杂比例的增加而增加(除了M-BiOI-6), M-BiOI-5的
光催化剂的重复性测试在工业生产中是非常重要的, 它可以降低操作费用, 提高经济效益[24].结合前面测试结果分析, 本文认为M-BiOI-5具有最优的催化性能, 因此对M-BiOI-5光催化测试前后进行XRD表征, 结果如图 10所示.由图 10可知, M-BiOI-5在降解罗丹明B前后的XRD图并无太大变化, 说明在降解过程中催化剂的结构并没有受到破坏, 反应过程中催化剂并没有参与化学反应. 图 11是M-BiOI-5在可见光条件下3次循环降解罗丹明B的降解曲线图.由图 11可以看出, 在3次循环降解后, 其光催化降解效率并无明显降低, 由此可以认为M-BiOI-5具有很好的循环性, 且有很好的工业应用价值.
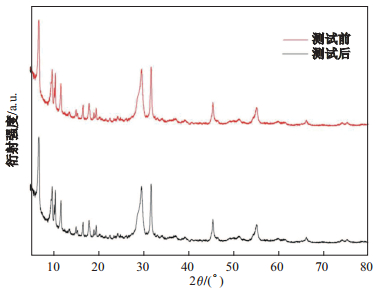
|
图 10 M-BiOI-5光催化性能测试前和测试后的XRD图 Fig.10 XRD patternsof M-BiOI-5 before and after photocatalytic performance testing |
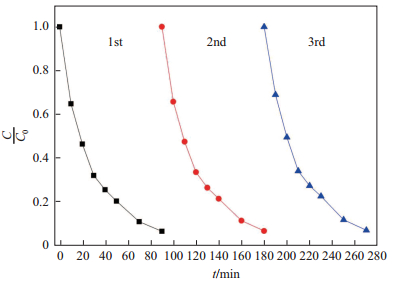
|
图 11 可见光条件下M-BiOI-53次循环降解罗丹明B的降解曲线图比较 Fig.11 Degradation curve for three cycles of degrading RhB for M-BiOI-5 under visible light |
采用Mulliken理论分别对MIL-125(Ti)和BiOI的能带进行计算[25-26], 公式为
| $ \begin{align*} &E_{\rm VB}=X - E_{\rm e}+ 0.5 E_{\rm g}, \\ &E_{\rm CB}=E_{\rm VB}- E_{\rm g}, \end{align*} $ |
其中,
图 12所示, BiOI的导带能级的位置(0.58eV)比MIL-125(Ti)的导带能级(
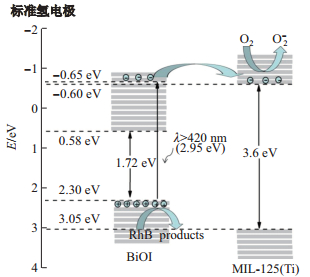
|
图 12 MIL-125(Ti)/BiOI异质结结构光催化剂机理图 Fig.12 Mechanism of photocatalyst of heterostructure for MIL-125(Ti)/BiOI |
利用一步共沉淀法合成了不同掺杂比例的MIL-125(Ti)/BiOI异质结光催化剂.根据XRD谱图可知, M-BiOI-
| [1] |
SE-NA KIM, JUN KIM, HEE-YOUNG KIM, et al. Adsorption/catalytic properties of MIL-125 and NH2-MIL-125[J]. Catalysis Today, 2013, 204: 85-93. DOI:10.1016/j.cattod.2012.08.014 |
| [2] |
CAO J, XU B U, LIN H L, et al. Chemical etching preparation of BiOI/BiOBr heterostructu-res with enhanced photocatalytic properties fororganic dye removal p[J]. Chem EngJ, 2012, 185/186: 91-99. DOI:10.1016/j.cej.2012.01.035 |
| [3] |
LI H L, EDDAOUDI M, O'KEEFFE M, et al. Design and synthesis of an exceptionally stable and highly porous metal-organic framework[J]. Nature, 1999, 402: 276-279. DOI:10.1038/46248 |
| [4] |
NASALEVICH M A, VAN DER VEEN M, KAPTEIJN F, et al. Metal-organic frameworks as heterogeneous photocatalysts:Advantages and challenges[J]. CrystEngComm, 2014, 16: 4919-4926. DOI:10.1039/C4CE00032C |
| [5] |
ALVARO M, CARBONELL E, FERRER B, et al. Semiconductor behavior of a metal-organic framework (MOF)[J]. Chemistry-A European Journal, 2007, 13: 5106-5112. DOI:10.1002/(ISSN)1521-3765 |
| [6] |
HASAN Z, JEON J, JHUNG S H. Adsorptive removal of naproxen and clofibric acid from watarusing metalorganic frameworks[J]. Journal of Hazardous Materials, 2012, 209: 151-157. |
| [7] |
DU J J, YUAN Y P, SUN J X, et al. New photocatalysts based on MIL-53 metal-organic frameworks for the decolorization of methylene blue dye[J]. Journal of Hazardous Materials, 2011, 190: 945-951. DOI:10.1016/j.jhazmat.2011.04.029 |
| [8] |
XIA J, YIN S, LI H M, et al. Enhanced photocatalytic activityof bismuth oxyiodine (BiOI) porousmicrospheressynthesizedvia reactable ionicliquid-assistedsolvothermaI method[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2011, 387(123): 23-28. |
| [9] |
LÜ Y H, LIU H, ZHANG W, et al. Room-temperature synthesis and high visible-light-induced photocatalytic activity of AgI/BiOI composites[J]. Journal of Environmental Chemical Engineering, 2013(1): 526-533. |
| [10] |
HE Z Q, XIE L, TU J J, et al. Visible light-induced degradation of phone lover iodine-d-opedtitanium dioxide modified with platinum:Role of platinum and there action mechanism[J]. Journal of Chemical Physics, 2010, 114(1): 526-532. |
| [11] |
CHAI I H, TANG X M, WEAVERS I K. Kinetics and mechanism of photo activated periodate re-action with 4-chlorophenol in acidic soIution[J]. Environmental Science & Technology, 2004, 38(24): 6875-6880. |
| [12] |
FUJISHMA A, HONDA K. Photolysis-decomposition of water at surface of an irradiated semiconductor[J]. Nature, 1972, 238: 37-38. DOI:10.1038/238037a0 |
| [13] |
SHI X J, CHEN X, CHEN X L, et al. PVP assisted hydrothermal synthesis of Bi-OBr hierarchical nanostructures and high photocatalytic capacity[J]. Chemical Engineering Journal, 2013, 222: 120-127. DOI:10.1016/j.cej.2013.02.034 |
| [14] |
HOFFMANN M R, MARTIN S T, CHOI W, BAHNEMANN D W. Environmental applications of semicon-ductor photocatalysis[J]. Chemical Reviews, 1995, 95: 69-96. DOI:10.1021/cr00033a004 |
| [15] |
CHEN X, MAO S S. Titanium dioxide nanomaterials:synthesis, properties, modifications, and applications[J]. Chemical Reviews, 2007, 107(7): 2891-2959. DOI:10.1021/cr0500535 |
| [16] |
MAO Y B, WONG S S. Size and shape-dependent transformation of nanosized titanate into analogous Anatase Titania Nanostructures[J]. Journal of the American Chemical Society, 2006, 128(25): 8217-8226. DOI:10.1021/ja0607483 |
| [17] |
WANG W D, HUANG F Q, LIN X P. xBiOI-(1-x)BiOCl as efficient visible-light-driven photo-catalysts[J]. Scripta Materialia, 2007, 56(8): 669-672. DOI:10.1016/j.scriptamat.2006.12.023 |
| [18] |
XIA J X, YIN S, LI H M, et al. Self-assembly and enhanced photocata-lytic properties of BiOI hollow microspheres via a reactable Ionic Liquid[J]. Langmuir, 2011, 27(3): 1200-1206. DOI:10.1021/la104054r |
| [19] |
CAO J, XU B Y, LUO B D, et al. Novel BiOI/BiOBr heterojunctionphotocatalysts with enhanced visible light photocatalytic properties[J]. Catalysis Communicati-ons, 2011, 13: 63-68. |
| [20] |
HENDON C H, TIANA D, FONTECAVE M, et al. Engineering the optical res-ponse of the titanium-MIL-125 metal-organic framework through ligand functionalization[J]. Journal of the American Chemical Soceity, 2013, 135: 10942-10945. DOI:10.1021/ja405350u |
| [21] |
CHEN Y J, WEN M, WU Q S. Stepwise blossoming of BiOBr nanoplate-assembled microflowers and their visible-light photocatalytic activities[J]. CrystEngComm, 2011, 13: 3035-3039. DOI:10.1039/c0ce00955e |
| [22] |
LEI Y Q, WANG G H, SONG S Y, et al. Room temperature, te-mplate-free synthesis of BiOI hierarchical structures:Visible-light photocatalytic and ele-ctrochemical hydrogen storage properties[J]. Dalton Transactions, 2010, 39: 3273-3278. DOI:10.1039/b922126c |
| [23] |
YU C L, YU J C, FAN C F, et al. Synthesis and characterization of Pt/BiOI na-noplate catalyst with enhanced activity under visible light irradiation[J]. Materials Science and Engineering B, 2010, 166: 213-219. DOI:10.1016/j.mseb.2009.11.029 |
| [24] |
ROSS H, BENDIG J, HECHT S. Sensitized photo catalytical oxidation of terbutylazine[J]. Solar Energy Materials and Solar Cells, 1994, 33(4): 475-481. DOI:10.1016/0927-0248(94)90007-8 |
| [25] |
CHATTERJEE D, MAHATA A. Demineralization of organic Pollutants on the DyeModified TiO2 semiconductor particulate system using visible light[J]. Applied Catalysis Environmental, 2001, 33(2): 119-125. DOI:10.1016/S0926-3373(01)00170-9 |
| [26] |
MOSER J, GRAETZEL M. Photoxensitixed electron injection in colloidal semiconductors[J]. Journal of the American Chemical Society, 1984, 106: 6557-6564. DOI:10.1021/ja00334a017 |




